
TAKEDA FURTHER ENABLES HEMOPHILIA A PERSONALIZED CARE WITH LAUNCH OF MYPKFIT® SOFTWARE FOR ADYNOVATE ® [ANTIHEMOPHILIC FACTOR (RECOMBINANT), PEGYLATED]
Cambridge, Mass. and Osaka, Japan, December 2, 2020 – Takeda Pharmaceutical Company Limited (TSE:4502/NYSE:TAK) (“Takeda”) today announced the U.S. availability of myPKFiT® for ADYNOVATE® [Antihemophilic Factor (Recombinant), PEGylated], a web-based software and mobile application that is the first and only pharmacokinetic (PK)-dosing software approved by the U.S. Food and Drug Administration (FDA) for hemophilia A patients 12 and older and weighing at least 29 kg treated with ADYNOVATE.1
The myPKFiT for ADYNOVATE software leverages a streamlined approach to estimating PK curves, a key measure for assessing drug exposure over time.2,3 With this software, healthcare professionals (HCPs) can estimate a full PK curve with as few as two measurable blood samples, compared to 9 to 11 as recommended by guidelines from the International Society on Thrombosis and Haemostasis (ISTH).2,4 HCPs can then create a visual depiction of the PK curve using this patient information and sample data.2
|
|
“More than ever, hemophilia management is driven by personalization, as we attempt to take a customized approach to care for each individual patient,” said Dr. Cindy Leissinger, Professor and Director of the Louisiana Center for Bleeding and Clotting Disorders at Tulane University. “It’s important for healthcare professionals and patients to have access to treatment options that help them manage care and prevent bleeding. With the FDA approval of myPKFiT for ADYNOVATE, patients will have another option available to them as they work closely with their healthcare professional to tailor their treatment regimen to their needs.”
The myPKFiT for ADYNOVATE software for HCPs is accompanied by a patient app for Apple and Android smartphone devices that allows users to log and track bleeds and infusions while also estimating their current level of factor VIII (FVIII), allowing them to structure their lifestyle and activities to align with their relevant level of FVIII coverage. 2,5
“myPKFiT for ADYNOVATE underscores our commitment to delivering personalized approaches to help manage hemophilia A,” said Michael Denne, Vice President of U.S. Medical, Hematology and Rare Disease at Takeda. “The ability to adjust activity and lifestyle decisions based on estimated FVIII coverage is a step toward educating patients so their treatment can be personalized to their needs. That, for us, is the goal for PK-focused dosing to allow people to get the most out of their treatment regimen.”
myPKFiT for ADYNOVATE was granted approval by the FDA in January 2020.1 Similarly, the FDA granted approval to myPKFiT for ADVATE® [Antihemophilic Factor (Recombinant)], a web-based software that aids healthcare professionals in personalizing a patient’s prophylaxis dose and schedule of ADVATE, in 2017 and the patient application became available in August 2018.6
myPKFiT is Rx only. For safe and proper use of the software and mobile app, please refer to the complete instructions for use in the respective User Manuals.
myPKFiT for ADVATE [Antihemophilic Factor (Recombinant)] and ADYNOVATE [Antihemophilic Factor (Recombinant), PEGylated] Intended Use
- The myPKFiT software is intended for use by licensed healthcare professionals (HCPs) who are familiar with hemophilia care. The myPKFiT software can be used to generate ADVATE or ADYNOVATE dosage amount and frequency recommendations using an individual patient’s age and body weight information and local laboratory FVIII one-stage clotting activity measurements of sparse samples collected from that patient. For ADVATE, myPKFiT is intended to be used with patients 16 years of age or older and body weight of 45 kg or greater. For ADYNOVATE, myPKFiT is intended to be used with patients 12 years of age or older and body weight of 29 kg or greater.
- For ADVATE, a minimum of two sparse sampling points are required at the recommended 3-4 hours (± 30 minutes) and at 24-32 hours (±1 hour) post-infusion. For ADYNOVATE, a minimum of two sparse sampling points are required at the recommended 3 hours (± 30 minutes) and at 48 hours (±2 hours) and/or 72 hours (±2 hours).
- HCPs will also be able to evaluate various prophylaxis dose regimens tailored to an individual patient’s needs and treatment plan.
- The software output may be used to guide decisions on appropriate ADVATE or ADYNOVATE dose and infusion intervals to maintain FVIII activity levels at or above a user specified minimum FVIII activity level between 1% to 3% above natural baseline for an individual patient in accordance with the FDA-approved dosing recommendations provided in the ADVATE or ADYNOVATE Prescribing Information (PI).
- myPKFiT should only be used to evaluate prophylactic dosing regimens for hemophilia A patients treated with ADVATE or ADYNOVATE, as per their respective Prescribing Information (PI).
- myPKFiT is not indicated for the treatment of von Willebrand disease. myPKFiT should not be used for patients who have developed neutralizing antibody (inhibitor) to FVIII products.
ADYNOVATE [Antihemophilic Factor (Recombinant), PEGylated] and ADVATE [Antihemophilic Factor (Recombinant)] Important Information
Indications and Limitation of Use
ADYNOVATE and ADVATE are each a human, recombinant antihemophilic factor indicated in children and adults with hemophilia A (congenital factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes.
- Perioperative management.
- Routine prophylaxis to reduce the frequency of bleeding episodes.
ADYNOVATE and ADVATE are not indicated for the treatment of von Willebrand disease.
DETAILED IMPORTANT RISK INFORMATION
CONTRAINDICATIONS
- ADYNOVATE is contraindicated in patients with prior anaphylactic reaction to ADYNOVATE, to its parent molecule, ADVATE, mouse or hamster protein, or to its excipients (e.g. Tris, mannitol, trehalose, glutathione, and/or polysorbate 80).
- ADVATE is contraindicated in patients who have life-threatening hypersensitivity reactions, including anaphylaxis, to mouse or hamster protein or other constituents of the product.
WARNINGS & PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions are possible with ADYNOVATE and ADVATE. Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with ADVATE (which is the parent molecule of ADYNOVATE) and other recombinant antihemophilic factor VIII products. Symptoms include dizziness, paresthesia, rash, flushing, facial swelling, urticaria, dyspnea, pruritus and vomiting. Early signs of hypersensitivity reactions that can progress to anaphylaxis may include angioedema, chest-tightness, dyspnea, wheezing, urticaria and pruritus. Immediately discontinue administration of ADYNOVATE or ADVATE and initiate appropriate emergency treatment if hypersensitivity reactions occur.
Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to factor VIII can occur following administration of ADYNOVATE or ADVATE. Inhibitors have been reported following administration of ADVATE predominantly in previously untreated patients (PUPs) and previously minimally treated patients (MTPs). Monitor all ADYNOVATE and ADVATE patients regularly for the development of factor VIII inhibitors by appropriate clinical observation and laboratory tests. Perform an assay that measures factor VIII inhibitor concentration if the plasma factor VIII level fails to increase as expected, or if bleeding is not controlled with expected dose of ADYNOVATE or ADVATE.
ADVERSE REACTIONS
- ADVATE Serious Adverse Reactions: Serious adverse reactions seen with ADVATE are hypersensitivity reactions, including anaphylaxis, and the development of high-titer inhibitors necessitating alternative treatments to factor VIII.
- ADVATE Common Adverse Reactions (>5% of subjects): The most common adverse reactions observed in clinical trials were pyrexia, headache, cough, nasopharyngitis, arthralgia, vomiting, upper respiratory tract infection, limb injury, nasal congestion and diarrhea.
- ADYNOVATE Common Adverse Reactions (>1% of subjects): The most common adverse reactions reported in clinical studies were headache and nausea.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
For additional safety information, click here for ADYNOVATE Prescribing Information, or here for ADVATE Prescribing Information.
About Hemophilia A
Hemophilia A, the most common type of hemophilia, is a rare bleeding disorder that causes longer-than-normal bleeding due to lack of clotting factor VIII in the blood.7 The severity of hemophilia A is determined by the amount of factor in the blood, with more severity associated with lower amounts of factor.8 More than half of patients with hemophilia A have the severe form of the condition.8 Hemophilia primarily affects males, with an incidence of one in 5,000 male births in the United States. 9
|
Contacts: Japanese Media Kazumi Kobayashi +81 (0) 3-3278-2095 |
Media outside Japan David Murdoch +1 781-482-1741 |
References
- U.S. Food and Drug Administration. myPKFiT for ADYNOVATE Healthcare Professionals and Patients Mobile Application Supplement Approval. January 2, 2020.
- myPKFiT for Healthcare Professionals v3.3 User Manual. Takeda, Inc. 2020.
- Gustafson DL and Bradshaw-Pierce EL. Fundamental Concepts in Clinical Pharmacology. M. Hidalgo et al. (eds.), Principles of Anticancer Drug Development, Cancer Drug Discovery and Development, DOI 10.1007/978-1-4419-7358-0_2. Accessible at: http://media.axon.es/pdf/83236_2.pdf
- Lee M et al. "Scientific and Standardization Committee Communication The Design and Analysis of Pharmacokinetic Studies of Coagulation Factors." ISTH Website. https://c.ymcdn.com/sites/www.isth.org/resource/group/d4a6f49a-f4ec-450f-9e0f-7be9f0c2ab2e/official_communications/fviiipharmaco.pdf.
- myPKFiT for Patients Mobile Application v2.1 User Manual. Takeda, Inc. 2020.
- “Shire receives FDA clearance for myPKFiT™ for Advate® [Antihemophilic Factor (Recombinant)] to help personalize care for hemophilia A.” Takeda Newsroom. https://www.takeda.com/newsroom/shire-news-releases/2017/abhvp5/. Accessed February 2020.
- World Federation of Hemophilia. “What is hemophilia?” World Federation of Hemophilia website. http://www.wfh.org/en/page.aspx?pid=646. Accessed February 2020.
- National Hemophilia Foundation. “Hemophilia A.” National Hemophilia Foundation website. https://www.hemophilia.org/Bleeding-Disorders/Types-of-Bleeding-Disorders/Hemophilia-A. Accessed February 2020.
- Centers for Disease Control and Prevention. “Hemophilia Data & Statistics”. CDC website. https://www.cdc.gov/ncbddd/hemophilia/data.html Accessed February 2020.
+++
About Takeda Hematology
Following its recent acquisition of Shire, Takeda is a leader in hemophilia with the longest heritage and market-leading portfolio, backed by established safety and efficacy profiles with decades of real-world experience. We have 70+ years driving innovation for patients9 and a broad portfolio of 11 products across multiple bleeding disorders.10 Our experience as leaders in hematology means we are well prepared to meet today’s needs as we pursue future developments in the care of bleeding disorders. Together with the hematology community, we are raising expectations for the future, including earlier diagnosis, earlier and full protection against bleeds, and more personalized patient care.
About Takeda Pharmaceutical Company
Takeda Pharmaceutical Company Limited (TSE:4502/NYSE:TAK) is a global, values-based, R&D-driven biopharmaceutical leader headquartered in Japan, committed to bringing Better Health and a Brighter Future to patients by translating science into highly-innovative medicines. Takeda focuses its R&D efforts on four therapeutic areas: Oncology, Rare Diseases, Neuroscience, and Gastroenterology (GI)and. We also make targeted R&D investments in Plasma-Derived Therapies and Vaccines. We are focusing on developing highly innovative medicines that contribute to making a difference in people's lives by advancing the frontier of new treatment options and leveraging our enhanced collaborative R&D engine and capabilities to create a robust, modality-diverse pipeline. Our employees are committed to improving quality of life for patients and to working with our partners in healthcare in approximately 80 countries.
For more information, visit https://www.takeda.com.
Important Notice
For the purposes of this notice, “press release” means this document, any oral presentation, any question and answer session and any written or oral material discussed or distributed by Takeda Pharmaceutical Company Limited (“Takeda”) regarding this release. This press release (including any oral briefing and any question-and-answer in connection with it) is not intended to, and does not constitute, represent or form part of any offer, invitation or solicitation of any offer to purchase, otherwise acquire, subscribe for, exchange, sell or otherwise dispose of, any securities or the solicitation of any vote or approval in any jurisdiction. No shares or other securities are being offered to the public by means of this press release. No offering of securities shall be made in the United States except pursuant to registration under the U.S. Securities Act of 1933, as amended, or an exemption therefrom. This press release is being given (together with any further information which may be provided to the recipient) on the condition that it is for use by the recipient for information purposes only (and not for the evaluation of any investment, acquisition, disposal or any other transaction). Any failure to comply with these restrictions may constitute a violation of applicable securities laws.
The companies in which Takeda directly and indirectly owns investments are separate entities. In this press release, “Takeda” is sometimes used for convenience where references are made to Takeda and its subsidiaries in general. Likewise, the words “we”, “us” and “our” are also used to refer to subsidiaries in general or to those who work for them. These expressions are also used where no useful purpose is served by identifying the particular company or companies.
Forward-Looking Statements
This press release and any materials distributed in connection with this press release may contain forward-looking statements, beliefs or opinions regarding Takeda’s future business, future position and results of operations, including estimates, forecasts, targets and plans for Takeda. Without limitation, forward-looking statements often include words such as “targets”, “plans”, “believes”, “hopes”, “continues”, “expects”, “aims”, “intends”, “ensures”, “will”, “may”, “should”, “would”, “could” “anticipates”, “estimates”, “projects” or similar expressions or the negative thereof. Forward-looking statements in this document are based on Takeda’s estimates and assumptions only as of the date hereof. Such forward-looking statements do not represent any guarantee by Takeda or its management of future performance and involve known and unknown risks, uncertainties and other factors, including but not limited to: the economic circumstances surrounding Takeda’s global business, including general economic conditions in Japan and the United States; competitive pressures and developments; changes to applicable laws and regulations; the success of or failure of product development programs; decisions of regulatory authorities and the timing thereof; fluctuations in interest and currency exchange rates; claims or concerns regarding the safety or efficacy of marketed products or product candidates; the timing and impact of post-merger integration efforts with acquired companies; and the ability to divest assets that are not core to Takeda’s operations and the timing of any such divestment(s), any of which may cause Takeda’s actual results, performance, achievements or financial position to be materially different from any future results, performance, achievements or financial position expressed or implied by such forward-looking statements. For more information on these and other factors which may affect Takeda’s results, performance, achievements, or financial position, see “Item 3. Key Information—D. Risk Factors” in Takeda’s most recent Annual Report on Form 20-F and Takeda’s other reports filed with the U.S. Securities and Exchange Commission, available on Takeda’s website at: https://www.takeda.com/investors/reports/sec-filings/ or at www.sec.gov. Future results, performance, achievements or financial position of Takeda could differ materially from those expressed in or implied by the forward-looking statements. Persons receiving this press release should not rely unduly on any forward-looking statements. Takeda undertakes no obligation to update any of the forward-looking statements contained in this press release or any other forward-looking statements it may make, except as required by law or stock exchange rule. Past performance is not an indicator of future results and the results of Takeda in this press release may not be indicative of, and are not an estimate, forecast or projection of Takeda’s future results.
###
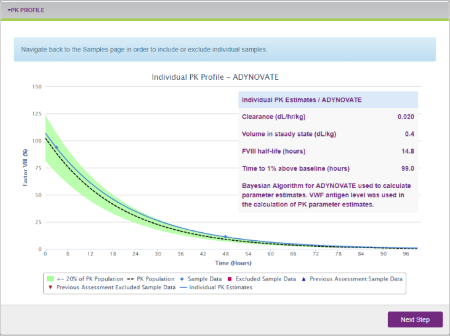 Sample PK Profile on the myPKFiT for ADYNOVATE HCP software.2
Sample PK Profile on the myPKFiT for ADYNOVATE HCP software.2